Welcome to
Dr. Debojyoti De
Assistant Professor
Department of Biotechnology
National Institute Of Technology, Durgapur
Debojyoti De graduated from Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, South Korea with PhD in Molecular Cell Biology with specialization in Regenerative Medicine. He is a Stem cell Biologist by training and possesses a vast experience in handling multidimensional genomics and epigenetics data, especially in dissecting complex biological mechanisms of cellular fate change and also to understand metabolic disorders. After the completion of his PhD degree, he worked as a Post-Doctoral Fellow and subsequently as Research Professor at Sungkyunkwan University School of Medicine.
Current Position
Assistant Professor, Department of Biotechnology, National Institute of Technology Durgapur (Jan 2019).

Our Interest
We are actively pursuing research on metabolic disorders as well as cellular fate change (Trans-differentiation /Reprogramming) by applying multi omics approach along with several Machine Learning tools to understand and decipher various hidden evolutionary semantics and to develop therapeutic interventions. On the other hand we also use several deep learning approaches for predicting novel usage of small molecules as therapeutic agents.
The Laboratory of Cellular Differentiation and Metabolic Disorder at NIT Durgapur explores metabolic disorders at various levels:

Diabesity :
Type II diabetes and obesity are intricately linked physiologically and hence they are often described in conjugation as ‘diabesity’. Primarily characterized by hyperinsulinemia and progressive insulin resistance the molecular link connecting obesity and Type II diabetes is a new therapeutic target in managing diabesity. Towards this we use a multi-omics approach in combination with AI/ML tools to understand the pathophysiology as well as to design novel therapy.
We aim to decode
The molecular molecular link connecting obesity with Type II diabetes, and hyperinsulinemia with PCOS
The functional implication of genomic variants in manifestation of diabesity
Novel therapeutics through drug repositioning and promoting brown adipogenesis
The role of gut microbiome towards predisposition and manifestation of diabesity
To accomplish this, we effectively leverage both computational and molecular and cell biology techniques.
Network Biology :
Genes associated with phenotypes related to diabesity tend to remain proximal to each other within gene interaction network. It further suggests that even the potential drug targets associated to diabesity might also cluster within the network. Our laboratory aims to dissect some of these intricate interactions by computing several network parameters, thereby accessing their relevance in disease manifestation as well as therapy.


Gut Microbiome and Diabesity :
Recently contribution of gut microbiome is well recognized in many disorder including obesity and diabetes. Our group explores the involvement of different taxa and their molecular pathways in infliction of diabesity. We further explore the cross talk between gut microbial metabolomics space and host signaling axis.
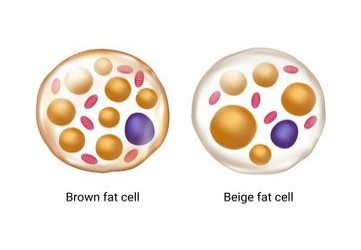
Brown adipogenesis :
Brown and beige fats are involved in thermogenesis and are highly dedicated in dissipating chemical energy in the form of heat. There is great hope that prevalence these cells can be promoted to therapeutically combat obesity, insulin resistance and type 2 diabetes. We explore ways for cellular fate change in order to augment brown and beige adipocyte population in vitro and in vivo. We employ several multi-omics approach in combination with network biology, computation and cell biology to achieve this augmentation.

Polycystic ovary syndrome :
PCOS a common hormonal disorder among women of reproductive age has also been associated with hyperinsulinemia. Our endeavor to understand its pathophysiology and devise effective therapy is based on studying genomic, transcriptomic and genomic variants and chromosomal interaction in 3D space.
Publications
Chanda, D., and De, D. (2024). Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut Microbes.
DOI: https://doi.org/10.1080/19490976.2024.2304900 (Corresponding author)
S Dutta, T Natoli, D Chanda, Sindhuja R, D De*. 2022. A Network based Efficient Drug Repurposing Strategy for Targeting Diabesity. Genes and Diseases. DOI: 10.1016/j.gendis.2022.02.015 (Corresponding author)
P Mandal, D De, D U Im, S H Um, K K Kim. 2020. Exosome-Mediated Differentiation of Mouse Embryonic Fibroblasts and Exocrine Cells into β-Like Cells and the Identification of Key miRNAs for Differentiation. Biomedicines. 8(11): 485. DOI: 10.3390/biomedicines8110485
JA Lee, J An, J Taniguchi, G Kashiwazaki G N Pandian, N Parveen, T M Kang, H Sugiyama*, D De* K K Kim*. 2020. Targeted epigenetic modulation using a DNA‐based histone deacetylase inhibitor enhances cardiomyogenesis in mouse embryonic stem cells. Journal of Cellular Physiology.
DOI: https://doi.org/10.1002/jcp.30140
(Co-corresponding Author)
P Mandal, D De, K Yun, K K Kim. 2020. Improved differentiation of human adipose stem cells to insulin-producing β-like cells using PDFGR kinase inhibitor Tyrphostin9. Biochem Biophys Res Commun. 533(1): 132-138. DOI: 10.1016/j.bbrc.2020.08.090
JA Lee, J An, TM Kang, D De*, KK Kim*. Discovery of natural compounds promoting cardiomyocyte differentiation. 2019. Stem Cells and Development. 28(1):13-27. DOI: 10.1089/scd.2018.0153 (Co-corresponding Author)
V Bansal*, D De*, J An, TM Kang, HJ Jeong, JS Kang, KK Kim. 2019. Chemical induced conversion of mouse fibroblasts and human adipose-derived stem cells into skeletal muscle-like cells. Biomaterials. 193:30-46. DOI: 10.1016/j.biomaterials.2018.11.037
(Co-First Author)
D De, D Halder, I Shin, K K Kim. 2017. Small molecule-induced cellular conversion. Chem Soc Rev. 46(20): 6241-6254. DOI: https://doi.org/10.1039/C7CS00330G
A Saha, S Kizaki, D De, M Endo, K K Kim, H Sugiyama. 2016. Examining cooperative binding of Sox2 on DC5 regulatory element upon complex formation with Pax6 through excess electron transfer assay. Nucleic Acids Res. 44(14): e125.
D Halder, GE Chang, D De, E Cheong, K K Kim, I Shin. 2015. Combining Suppression of Stemness with Lineage-Specific Induction Leads to Conversion of Pluripotent Cells into Functional Neurons. Chem Biol. 22(11): 1512-1520. DOI: 10.1016/j.chembiol.2015.10.008
S Yamamoto , *D De, K Hidaka, K K Kim, M Endo, H. Sugiyama. Single Molecule Visualization and Characterization of Sox2-Pax6 Complex Formation on a Regulatory DNA Element Using a DNA Origami Frame. Nano Lett. 14(5): 2286-2292. DOI: https://doi.org/10.1021/nl4044949 (Co-First Author)
D De, MH Jeong, YE Leem, DI Svergun, DE Wemmer, JS Kang, KK Kim, SH Kim. 2014. Inhibition of master transcription factors in pluripotent cells induces early stage differentiation. Proc Natl Acad Sci USA. 111(5): 1778-1783. DOI: https://doi.org/10.1073/pnas.1323386111
D Dutta, D De, S Chaudhuri, SK Bhattacharya. 2005. Hydrogen production by Cyanobacteria. Microb Cell Fact. 4:36. DOI: https://doi.org/10.1186/1475-2859-4-36.
D De, D Dutta, M Kundu, S Mahato, MT Schiavone, S Chaudhuri, A Giri, V Gupta, SK Bhattacharya. 2005. Inactive enzymatic mutant proteins (phosphoglycerate mutase and enolase) as sugar binders for ribulose-1,5-bisphosphate regeneration reactors. Microb Cell Fact. 4(1):5. DOI: https://doi.org/10.1073/pnas.1323386111
S Mahato, D De, D Dutta, M Kundu, S Bhattacharya, MT Schiavone, SK Bhattacharya. 2004. Potential use of sugar binding proteins in reactors for regeneration of CO2 fixation acceptor D-Ribulose-1,5-bisphosphate. Microb Cell Fact. 3(1):7. DOI: https://doi.org/10.1186/1475-2859-3-7
Patents
D De and K K Kim. Novel glia-like cells differentiated from somatic cells, preparation method therefor, cocktail composition for preparing same, cell therapeutic agent for preventing or treating neurological disorders, comprising same, and method for preventing and treating neurological disorders by administering same. 2019. AU2019418187A1, Australia.
D De and KK Kim. Methods and compositions for the direct conversion to brown adipocyte-like cells using low molecular weight compounds. 2019. KR20200129274A, South Korea.
D De and KK Kim. Media composition for differentiation of somatic cell into neuron and differentiation method of somatic cell into neuron using said media composition. 2018. WO2018160028A1, Worldwide PCT.
D De and KK Kim. Media composition for differentiation of somatic cell into schwann cell and differentiation method of somatic cell into schwann cell using said media composition. 2017. KR20190063792A, South Korea.
D De, JA Lee, KK Kim. Composition comprising Ursinoic acid derivatives for inducing the differentiation of stem cell and uses thereof. 2016. KR101669368B1, South Korea.
D De, JA Lee, KK Kim. Composition comprising Lupinine derivatives for inducing the differentiation of stem cell and uses thereof. 2015. KR101669367B1, South Korea.
D De, HS Lee, KK Kim. Compositions comprising securinine for inducing the differentiation of cancer stem cell as an effective ingredient and uses thereof. 2015. KR101539205B1, South Korea.
D De, JS Kang, KK Kim. Composition comprising SKP protein for inducing the differentiation of stem cell and uses thereof. 2014. KR101465354B1, South Korea.
Lab Members
Current Members
Alumini
Harshada K
(M.Tech)
Research interest: Genomic variants associated with Obesity and understanding their contributory effect.
Present position: Associate analyst in Zifo RnD, Chennai.
Soumalya Bose
(M.Tech)
Reserach interest: Medical Technologies & Wireless Communications, Image and Signal Processing, AI and Computational Modelling.
Present position: Master’s and research assistant in Communications and Multimedia Engineering, University of Erlangen-Nuremberg, Germany.
Monojit Kamilya
(M.Tech)
Research interest: Multi’Omics and network biology using machine learning and deep learning.
Subhajit Dutta
(Project JRF)
Research interest: Understanding pathophysiology of Obesity and Type 2 Diabetes using systems- and network biology.
Sritama Srakar
(M.Sc)
Research interest: Understanding pathophysiology of Obesity and Type 2 Diabetes using systems- and network biology.
Funding
The Laboratory of Cellular Differentiation & Metabolic Disorder is currently supported by the Start Up Research Grant from SERB, Department of Science & Technology (DST), Govt. of India, and an Intramural Research Ignition grant from National Institute of Technology Durgapur. The lab has also been awarded funding from Indian Council of Medical Research (ICMR) for supporting Clinical research in obesity and metabolic disorder.



Gallery










Collaborations
To Pursue interdisciplinary research we work together with
Dr. Ted Natoli
Broad Institute of MIT and Harvard
Dr. Ashish Bhattacharjee
National Institute of Technology
Durgapur
Prof. Kyeong Kyu Kim
CEO, Cellapeutics Bio and Director, Institute for Antimicrobial Resistance Research and Therapeutics